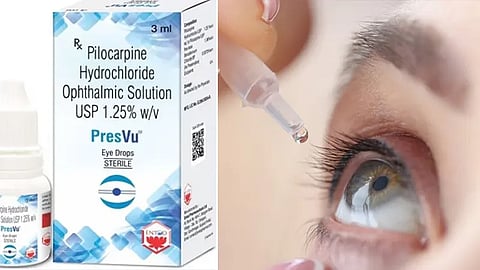

In a shocking turn of events, the Directorate General of Health Services (DGHS), under the Central Drugs Standard Control Organisation (CDSCO), recently suspended Mumbai-based ENTOD Pharmaceuticals Ltd’s licence to manufacture and market eyedrop PresVu. The pharmaceutical company launched PresVu with the claim that it can remove the need for reading glasses in just 15 minutes. To be precise, India's drug regulator suspended the company's licence to manufacture and market Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% w/v, branded as PresVu. Now, the question arises as to why India's drug regulator suspended PresVu's manufacturing & marketing. Below is what we know so far-
The answer is the misleading claims made by ENTOD Pharmaceuticals Ltd over ist eyedrop PresVu. The company had claimed that PresVu can prove beneficial in the treatment of presbyopia, an age-related vision condition.ENTOD Pharmaceuticals had received approval on 20 August 2024 to produce and market the eye drops for managing presbyopia in adults. However, a notice dated 4 September 2024 highlighted several unapproved claims made by the company in media releases.
The DGHS identified three specific instances where ENTOD allegedly overstated the product's capabilities, including claims that the eye drops could reduce the need for reading glasses and provide a non-invasive option to improve near vision within 15 minutes. The regulator clarified that these claims were not authorised, potentially misleading the public.
"...considering the public interest, the permission dated 20.08.2024 issued to manufacture and market of Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25% is hereby suspended till further order under the provisions of rule 84 of the New Drugs and Clinical Trials Rules, 2019, of the Drugs and Cosmetics Act, 1940,” read DCGI, Rajeev Singh Raghuvanshi’s letter.
CEO of Entod Pharmaceuticals, Nikhil K Masurkar had said, “PresVu has been created through years of research and development. Its approval by DCGI has marked a significant step in our mission to transform eye care in India. PresVu is a solution that can enhance the lives of millions by providing them with greater visual independence.”
Dr. Aditya Sethi said, “Presbyopia has long been treated with reading glasses, contact lenses and surgical measures. With PresVu, it can be treated effectively as the eye drop improves near vision within 15 minutes.”